Abstract
Introduction: Systemic AL amyloidosis is a plasma cell disease characterized by the deposition of insoluble amyloid fibrils causing organ dysfunction and death. Results from the ANDROMEDA study showed that the addition of daratumumab to bortezomib, cyclophosphamide, and dexamethasone (VCD) was associated with higher frequencies of hematologic complete response and survival free from major organ deterioration or hematologic progression. In our single-center retrospective real-life case series, we aimed to evaluate the role of AHCT in the era of Anti-CD38 monoclonal antibody based treatments for AL amyloidosis
Patients and Methods: One hundred and two consecutive patients newly diagnosed with AL amyloidosis at our center between 2007-2022 were included retrospectively. The F/M ratio was 50/52 The median age was 61 years (range:31-85), and the median follow-up was 11.8 months (range: 1-100 months). There were 68 (66%) patients with cardiac involvement and 79 (76.7%) patients with renal involvement at the time of diagnosis. Among all patients, 33% had stage 0-1, 60.2% ≥ stage 2 cardiac involvement. At the time of diagnosis, 61.2% of the patients had renal stage 2-3 disease. Fourthy-six patients (44.7%) patients had stages III and IV according to the Revised Mayo Clinic Staging System. Induction treatments that were used in our center were melphalan-dexamethasone(2007-2014), bortezomib-dexamethasone (2007-2022), VCD (2007-2022), Daratumumab-VCD (2018-2022) and up-front AHCT for transplant-eligible patients (2007-2022).
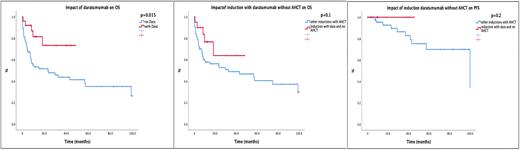
Results: Eighty of 102 patients (78.4%) underwent AHCT and post-transplant 41 patients achieved ≥VGPR but 43 patients deceased during post-transplant follow-up. Among patients who received daratumumab-based induction treatment without AHCT consolidation, 11/20 patients achieved ≥VGPR and none of these patients relapsed. In patients who received daratumumab-based induction treatment renal and cardiac responses were observed in 50% and 60%, respectively. Death was significantly lower among patients with Mayo Clinic Risk Stage 3-4 who received daratumumab (20% (3/15) vs 71% (22/31); p=0.002). Median overall survival was 56.8 months (95% CI 22.7-90.9) and the probability of 2-years OS was 56.2%. There were significant differences in median OS according to daratumumab administration (NR vs 23.3 mos; p=0.015) (Figure-1). Patients who had received daratumumab as an induction treatment without AHCT consolidation (n=20) have displayed prolonged OS compared to others (n=80) (NR vs 32.6 mos (95% CI 0-71.5); p=0.1) (Figure-2). There were no significant differences in 2-years PFS between the two groups but it's important to note that no progression was seen in the daratumumab group (Figure-3). If Cox proportional hazards model including Revised Mayo Clinic Stage, w/wo AHCT, and w/wo Daratumumab was used, only Daratumumab administration was associated with better PFS (HR: 18.8 (95% CI: 3.3-108.8); p=0.001).
Conclusion: From the prognostic point of view, our results based on real-world AL amyloidosis experience showed the improvement in OS with daratumumab-based treatments even in advanced stages of the disease. In addition, all patients rapidly achieved hematological response that deepened over time, and also cardiac and the renal response was also observed in a substantial proportion of our cohort. The data presented here add to the growing body of literature supporting the delayed use of AHCT in patients with AL amyloidosis.
Disclosures
Ozcan:Abbvie: Other: Travel expenses/accomodations, Research Funding; Acerta: Research Funding; Bayer: Research Funding; Janssen: Research Funding; MSD: Research Funding; Pfizer: Research Funding; Reedy's: Research Funding; Roche: Other: Travel expenses/accommodations, Research Funding; Takeda: Research Funding; Jazz: Other: Travel expenses/accommodations. Beksac:Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Oncopeptides: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal